Intrinsic imaging tips
Intrinsic signal optical imaging is a functional imaging modality where the reflectance of red light indicates active portions of cortex. It is used for many applications, including imaging individual barrels in rodent somatosensory cortex (e.g., Polley et al. 2004, Aronoff & Petersen 2007), maps in visual cortex (Blasdel & Salama 1986, Kalatsky & Stryker 2003), and the tonotopic organization in auditory cortex (Nelken et al. 2004, Kalatsky et al. 2005).
Here is a generic procedure:
- Image the surface vasculature with green illumination and take an image for later alignment.
- Switch to red illumination and grab frames during with and without stimulation.
- Average frames from the two conditions, then subtract the baseline image from the stimulated condition to determine the active areas of cortex.
Here are some tips for rigging this up in your own lab.
Use a high bit depth camera
You’ll be measuring a very small modulation, on the order of 10-4. Since 8-bit cameras have only 256 grey levels, it will be impossible to detect signals in single pairs of frames. However, a large amount of averaging will allow detection even with an 8-bit camera.
Determine the time course of the signal
The time course of the signal will vary a bit among cortical areas and model systems. You’ll want to know the time course so that you can design the appropriate acquisition parameters. For example, you don’t want to grab “baseline” or “blank” frames before the signal has actually returned to baseline. Similarly, you want to ensure that you’re grabbing stimulus evoked frames during the peak of the response.
Give pure oxygen
Being a metabolism-based hemodynamic signal, giving oxygen to the animal makes sense, whether using injectable or inhaled anesthetics. Typically this increases the amplitude of the signal.
Use bright, steady illumination
LEDs have really taken over in many areas of microscopy and imaging. They’re available in many different wavelengths, they’re cheap and easy to use, but best of all: they’re rock solid. Arc lamps and other sources can flicker and add terrible amounts of noise, where LEDs are perfectly flat. LEDs have gotten brighter, but they’re still not especially bright compared to traditional sources. So you might want to go with a bank of lights. This is an imaging application where you are not light limited, and in imaging the noise is lower the more photons you measure. So you want to set the illumination so that the camera is just below saturation.
Use a tandem lens macroscope
This is a classic tool for intrinsic imaging. Use two lenses on your camera, face to face. The ratio of their two focal lengths will be the magnification. This setup is nice because it lets in a lot of light, performs minimal magnification, and has a very shallow depth of field. Focus down from the surface vasculature 100-300 µm.
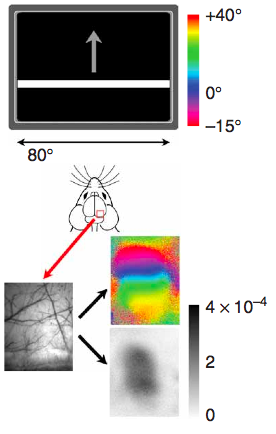
Special notes for Fourier-based intrinsic imaging (pictured above)
Kalatsky & Stryker (2003) introduced this paradigm to intrinsic imaging, where one variable of the stimulus is varied cyclically and then Fourier analysis is used to pick out the phase of response of each pixel in order to determine what part of the stimulus cycle stimulated that area of cortex. For this imaging, averaging is not used, so you’ll need a 12-bit camera, preferably with as much well depth as possible (Dalsa 1M30s and 1M60s are popular choices). In order to avoid artifacts, you’ll also want a frame scan camera, where the entire image frame is read at once, rather that line-by-line. Once the above requirements are fullfilled, go for the fastest acquisition available. Also, timing is very critical for this paradigm, so you need to ensure the stimulus and acquisition are precisely synchronized.
[…] are some of my favorites from quickly perusing the site: Printable bolt size charts, Tips on intrinsic optical imaging, Comparison of high NA, low mag objectives, and my favorite, Catalogs as textbooks. (I still […]
[…] computer is used, there may be multiple programs that need to talk to each other. In the first intrinsic imaging rig I built, I wrote my own OpenGL-based visual stimulation program which ran on the same computer […]
[…] makes coupling 35mm SLR lenses into optical setups fairly straightforward. I’m using it for a tandem lens macroscope. In the picture above I used ThorLabs part SM3A2. BTW, they also sell some F-mount adapters for […]
[…] Here is a prior Labrigger post with tips for intrinsic signal optical imaging. One of the key things is to use a high quality scientific camera. […]
Regarding the LED as light sources, would you please comment on what would be the ideal light delivery method? How is the Thor 4 wavelength LED box (http://www.thorlabs.com/newgrouppage9.cfm?objectgroup_id=3836) coupled with the liquid light guide for side illumination serving the purpose? Thanks a lot!
That solution can certainly work. It depends on your preparation, camera, and filters. You’ll want those all to work together to make sure you can get your camera close to saturation (at high light levels, the noise will be lower).
I’m using similar hardware from TL for the same purpose (their high power fiber-coupled LEDs together with the T-cube controllers). I use a custom bifurcated finer together with some fiber collimators to get two spots to equalise side-on illumination.
However, I find the LED/Controller combination not exceptionally power stable when used in a pulsed configuration:
I synchronise and cycle the pulsing of LEDs of different color (for quasi-simultaneous intrinsic/fluorescence imaging) with the exposure signal of my camera, which in turn is set to acquire out of phase with my visual stimulation monitor to prevent light contamination. The problem is that in pulsed mode I see a slight decay of the reflected light intensity over time that usually settles after a few seconds. I assume that this is a thermal issue. If you want to work in a pulsed regime, you should check this out first.
Best,T
[…] 12 or even 16 bits images). This is a technique that is extensively used in microscopy (notably intrinsic imaging for the […]
[…] More on intrinsic imaging… Yet more again… […]
Why we don’t use an intrinsic signal source for biomedical imaging ?
We do. It is sometimes used during brain surgery to map functional areas. Here are some references:
https://www.ncbi.nlm.nih.gov/pubmed/28630881
https://www.ncbi.nlm.nih.gov/pubmed/28018935
https://www.ncbi.nlm.nih.gov/pubmed/15771400